How do you know that the ultrasound findings that are on the final report are accurate? That is the whole reason that quality assurance is performed. There are several aspects of quality assurance. This article will not discuss cleaning as that falls under the safety arena. There are several issues we will look at here. Most of the time institutions think that all they have to do is a PM every six months and that is their QA program. Physician education, sonographer education and the accuracy of the machines and probes must be looked at to have a comprehensive program.
Physician education is one area that no one likes to talk about. Where were the doctors trained? How much contact did they have actually doing hands on ultrasound? Many programs do not have an active focus on ultrasound because there are tests and procedures to learn that have better reimbursement. This renders ultrasound to an educational byline. Then you need to look at the continuing education in ultrasound and licensing in the specialties. In South Carolina all MD’s are required to have 40 hours every two years. Of that, 75% has to be in their specialty. Each state has their own laws regarding MD requirements. Any physician who has a specialty or sub specialty in ultrasound should be actively seeking this ongoing training.
Sonographer education has the same questions as the physicians. Who is training them? It should be a sonographer with significant credentials and experience in a hospital setting. Are the sonographers credentialed with ARDMS or CCI? If you do not have a sonographer who has been to an accredited school and has their credentials then the end results of their exams may be called into question. Sonographers also have to obtain a number of continuing medical education (CME’s) to keep their credentials current. Many states now have law requiring sonographers to be credentialed in order to work. So you can see if you have a poorly trained doctor and a poorly trained sonographer that alone is a recipe for disaster.
In terms of quality assurance there is an internal way to solve this problem. It is called Peer Review. Historically peer review has only been for doctors. In the last ten years both the doctors and sonographers have been going through peer review by institutions who value quality assurance. If any of the centers are ICA, ACR, AIUM accredited then it is mandatory for them to have a quality assurance program. I have been through seven peer reviews when I worked in the academic world. All my peer reviews were done by physician committees and one consensus report was written and sent to the department head. Sonographer diagnostic acumen is not really addressed in this way. Again centers give sonographer an evaluation once a year and that is it. Those evaluations are NOT the same as a peer review. Nor do they address the same issues. Also evaluations should be confidential. Peer reviews for QA are not generally confidential.
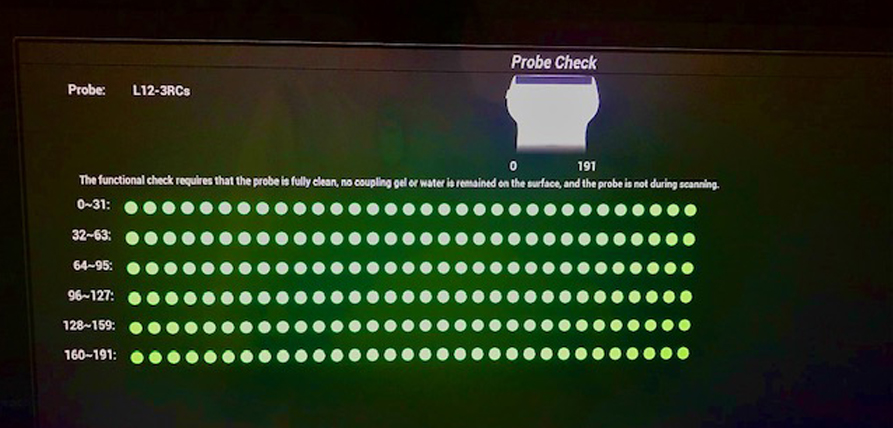
We saved the best for last. The most objective and to some extent easiest part of this process is the machine and the probe. The bulk of this is done during a preventive maintenance (PM) by an engineer who has special training to look for issues in detail. They have to check measurement accuracy. What is 1mm must measure 1mm. Gray scale accuracy is tested on an ATS standard Phantom. Accurate registration is very important. The probes are checked by eye and by task. In picture one there is a new tool used to check the probes. It is called probe check. You can see that the elements are numbered. Green is good and red is bad. This is very easy and takes a half a second to do per probe. This should be done once a week for each probe and documented. This information is valuable to the engineers when the PM time comes. Every function on the machine should be checked and documented at least once a month by the sonographer and documented. Visual inspections of all parts of the equipment should be performed every week to flag problems early. Special Doppler phantoms are used to check all Doppler functions. Fans are to be cleaned routinely to keep the machines functioning well.
The cords, connectors, probe membranes and strains need to be examined for defects. Tears on probe membranes or loose caps on transvaginal probes are serious concerns for patients and staff safety. Those issues are pathways for infection but also for shock hazards. Loss of pins and missing parts of the membrane in the connector ends will adversely affect your image as well. Cords are filled with many wires and if some of them break because they are crimped, twisted or run over repeatedly then you will experience image degradation. The only way to prove this is with consistent monitoring every week. Unless you are looking for it this may not be readily noticeable the way a large drop out would be.
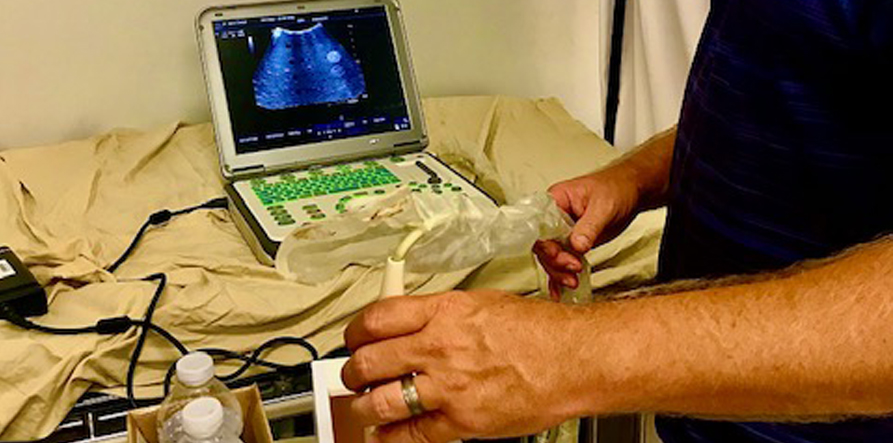
Mike Wilson using an ATS standard Phantom to check a curved probe.
You can see that this is a very important topic. Most centers concentrate on the machine and the probe but leave everything else behind. Naturally we would hope that correlative testing as well as surgical results are also documented to check your diagnoses. Our hope is to spark the realization that to do QA well you must invest time to protect your assets.
If you have any questions please call us at 843-566-1020.
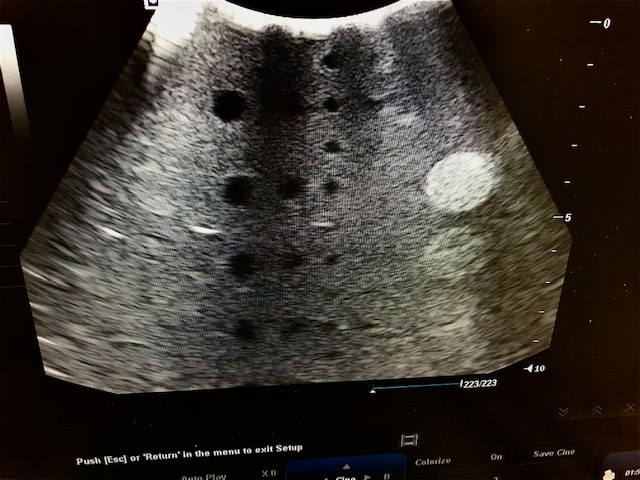
This is a close up of what Mike sees on the screen above. This shows three bad drop outs. This probe would need to be repaired and or replaced and is nondiagnostic. You can see some slight distortion of the circular areas . The gray areas are way too granular to show details of soft tissue. The echogenic areas to the left should be round small dots not echogenic dots arrows.